
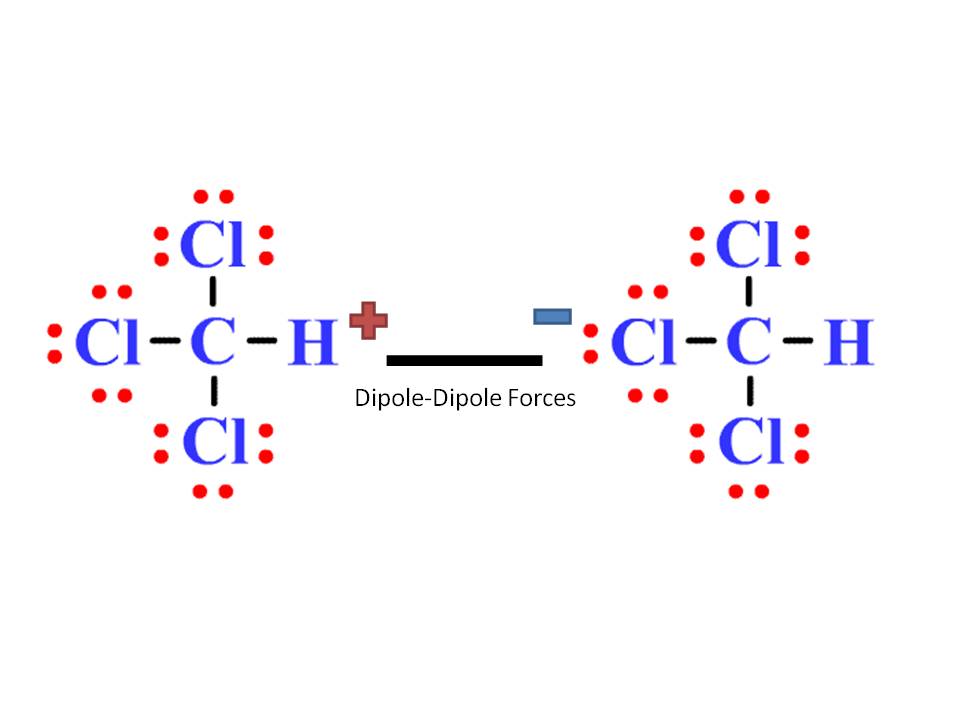
The attraction is between the hydrogen atom and the F, O, or N a neighboring molecule.

For example,-Select molecules will exhibit dipole dipole forces on each other (c) hydrogen bond Hydrogen bonding is a particularly Select type of dipole dipole attraction that occurs when a hydrogen atom is bonded to a small, -Select electronegative atom (including F, O,and N). The attraction occurs between the partial positive end of one polar molecule and the partial -Selectend of another polar molecule. For example, dispersion (b) dipole-dipole attraction A dipole-dipole force is the electrostatic attraction between two Select-dipoles. These forces are stronger in-Select- forces between-Selectmolecules will be stronger than dispersion forces between-Select molecules. Molecules must be very close together for these attractive forces to occur. Explanation: Factors that affects the strength of a dispersion force Distance between molecules. These two rapidly fluctuating dipoles thus result in a brief electrostatic にSelect-, between the two species. Factors that affects the strength of a dispersion force include : Distance between molecules, polarizability and the shape of the molecule. The presence of this dipole can then distort the electrons of a neighboring atom or molecule, producing an - Select - dipole. When the electrons within a molecule or atom are distributed asymmetrically about the nucleus, that molecule or atom will adopt a -SelectSelect-dipole. Larger and heavier atoms and molecules exhibit stronger dispersion forces than do smaller and lighter atoms and molecules. (a) dispersion force Dispersion forces are-Select-electrostatic attractions that occur due to the random electronic motion within all substances, -Select-, those that are nonpolar. Define the following and give an example of each.


 0 kommentar(er)
0 kommentar(er)
